Targeted Therapy in Oncology
Shots:
-
The oncology domain accounts for more than 50% of ongoing clinical trials. The global oncology drug market was valued at $185B in 2022 and is predicted to reach $205B by 2030 with a 13% CAGR
-
Targeted therapy targets specific genes and proteins to prevent them from growing and spreading. In 2022, the market size of Targeted Therapy accounted for $70B and is expected to hit $137.05B by 2032 with a 7% CAGR
-
PharmaShots brings an illuminating guide to Targeted Therapy in Oncology with this informative article
Over time, the oncology sector has witnessed phenomenal growth in treatment methodologies. Novel therapies are hitting the market like never before and with cutting-edge technological advances and studies, researchers are constantly looking for new aspects of treatments or modifying the old ones for oncology therapies. One such therapy that has been in practice for decades but has evolved with time and turned out to be the most effective is targeted therapy. It uses drugs to target specific genes and proteins that promote the survival and growth of cancer cells. Targeted therapy is used in the treatment of various types of cancer or combination with other cancer treatments, such as chemotherapy, or immunotherapy. The total global oncology spending increases substantially every year, and this trend is most likely to be seen in the long run.
How Targeted Therapy Works
To develop any targeted therapy, researchers identify a specific genetic modification that helps a tumor grow and change. Ideally, the drug’s target is a protein that is present only in cancer cells. The targets of this therapy are:
-
Too much of a certain protein in cancer cells
-
A specific protein in the cancer cell that is not present in normal cells
-
A mutated protein in a cancer cell
-
Gene changes that are not present in a normal cell
Once the target is identified, researchers develop a drug treatment that attacks it. The therapy is designed to perform mainly five functions on the cancer cells that include:
-
Blocking or turning off the signals that direct cancer cells to grow and divide
-
Preventing the cells from living longer than normal
-
Destroying cancer cells by triggering your immune system
-
Stop making new blood vessels to feed the cancer cells
-
Carrying toxins to the cancer cells
Types of Targeted Therapy
Targeted therapies are used in the treatment of many kinds of cancer. Some of the commonly used targeted therapies are:
-
Small-molecule Drugs: These drugs are administered orally as pills and are small enough to enter the cells without causing any immune response
-
Angiogenesis Inhibitors: These inhibitors block the formation of new blood vessels that feed and nourish the cancer cells. For example, bevacizumab
-
Monoclonal Antibodies: Researchers create antibodies that specifically target a certain antigen and then make exact copies of the antibody in the lab. These antibodies are called monoclonal antibodies. Some monoclonal antibodies come under target therapy as they aim for a specific target on a cancer cell, whereas other mAbs act like immunotherapy as they trigger the immune system to find and attack the cancer cells more effectively.
-
Proteasome Inhibitors: These inhibitors disrupt normal cell functions so that the cancer cells may die. For example, bortezomib is used in multiple myeloma.
-
Signal Transduction Inhibitors: They act by hindering cell signals to change the actions of the cancer cell. For example, imatinib is used in certain cases of chronic leukemia.
-
Tumor-agnostic Treatment: It uses the same drug to treat all types of cancer. This therapy treats specific genetic changes or biomarkers and is often referred to as tissue-agnostic therapy.
Indication-Specific Targeted Therapies
-
Melanoma: Approx. half of the melanomas have a mutation in the BRAF gene. There are many approved BRAF inhibitors as the mutations make great drug targets. Another targeted therapy inhibitor used in the treatment of melanoma is the MEK inhibitor. In the case of BRAF mutation, the two therapies can be combined for effective treatment.
-
Lymphoma: It is a cancer of the lymphatic system caused when there’s an overproduction of B cells, a type of white blood cell that protects the body against infections. There are many targeted drugs approved for the treatment of Lymphoma and some B-cell leukemia.
-
Breast Cancer: Nearly a quarter of breast cancer cases are due to the excess of a protein called human epidermal growth factor receptor 2 (HER2). The excess protein makes tumour cells grow. For the treatment of breast cancer, there are many approved target therapies.
-
Chronic Myeloid Leukaemia (CML): All cases of chronic myeloid leukaemia happen due to the formation of a gene called BCR-ABL, which leads to the production of an enzyme called BCR-ABL protein. The protein causes normal myeloid cells to behave as cancer cells. The targeted medicines used to treat CML are called tyrosine kinase inhibitors (TKI).
-
Colorectal Cancer: In this cancer, there’s often too much of a protein called epidermal growth factor receptor (EGFR). Those drugs that block EGFR may help stop or slow cancer growth. Alternatively, patients can also be administered a drug that blocks vascular endothelial growth factor (VEGF), a tumour-agnostic treatment that focuses on an NTRK fusion, and anti-angiogenesis therapy.
-
Lung Cancer: There are many approved targeted therapies to treat non-small cell lung cancer (NSCLC), which includes
-
EGFR inhibitors
-
Drugs targeting the EGFR exon 20 insertion
-
Drugs targeting NTRK fusion
-
Drugs targeting MET exon 14 skipping
-
Drugs targeting RET fusion
-
Drugs targeting BRAF V600E mutations
-
ALK inhibitors
-
Drugs targeting ROS1 fusion
-
Drugs targeting KRAS G12C mutations
Mentioned below are a few FDA-approved targeted therapies, for a detailed report reach out to us at connect@pharmashots.com
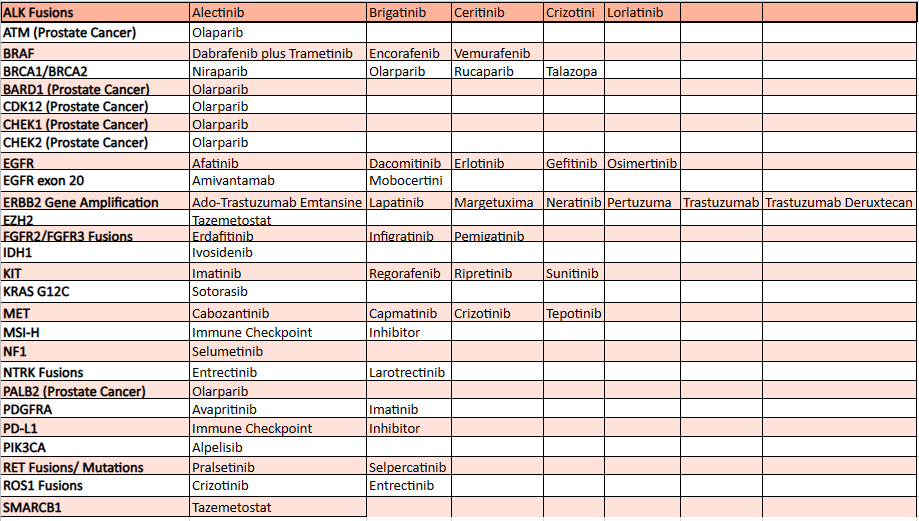
Limitations of Targeted Therapy
There are certain limitations of targeted therapy. The most common problem associated with targeted therapy is when the cancer cells become resistant to it. It happens when the target changes and the therapy cannot interact with it. In such cases, the best alternative to the problem is undergoing targeted therapy with other cancer treatments, such as chemotherapy or radiation therapy. Another drawback of targeted therapy is that in some cases it becomes really hard to develop drugs for some targets due to the target’s structure, cell function, or both.
Side Effects of Targeted Therapy
Targeted therapy when first developed was presumed to be free from adverse events as compared to chemotherapy but soon it was discovered that targeted therapies have a few downsides on the health of an individual. The common side effects of targeted therapy include:
-
High blood pressure
-
Fatigue
-
Mouth sores
-
Diarrhea
-
Liver problems
-
Loss of hair color
-
Fatigue
-
Skin problems
There are medicines to treat these side effects, and often these side effects go away after the treatment ends.
Conclusion
In the last few decades, major life science companies, all across the globe have expanded their focus on oncology. Targeted therapies are deemed to be the most effective cancer treatment. Researchers are keenly working on various small-molecule drugs and inhibitors, and thanks to them, there are various approved drugs and inhibitors available in the market. As far as mAbs are concerned, there are many approved drugs in oncology and plenty of them are in clinical trials waiting for approval. The shift in focus on developing drugs with high efficacy and fewer implications on normal cells has encouraged researchers to develop several oncological drugs.
Related Post: Role of Real-World Evidence in Pharma (RWE in Pharma)
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.